Is The Abbott Rapid Covid Test Fda Approved
While other approved tests could pop up Abbotts BinaxNow COVID-19 Home Test seems to be the best option for now. Curative executives said they had asked the FDA to revoke the tests emergency use authorization because the company has switched to offering tests provided by Abbott.

Abbott Obtains Ce Mark For Panbio Self Test To Detect Covid 19 Virus
It has been authorized by the FDA under an emergency use authorization.

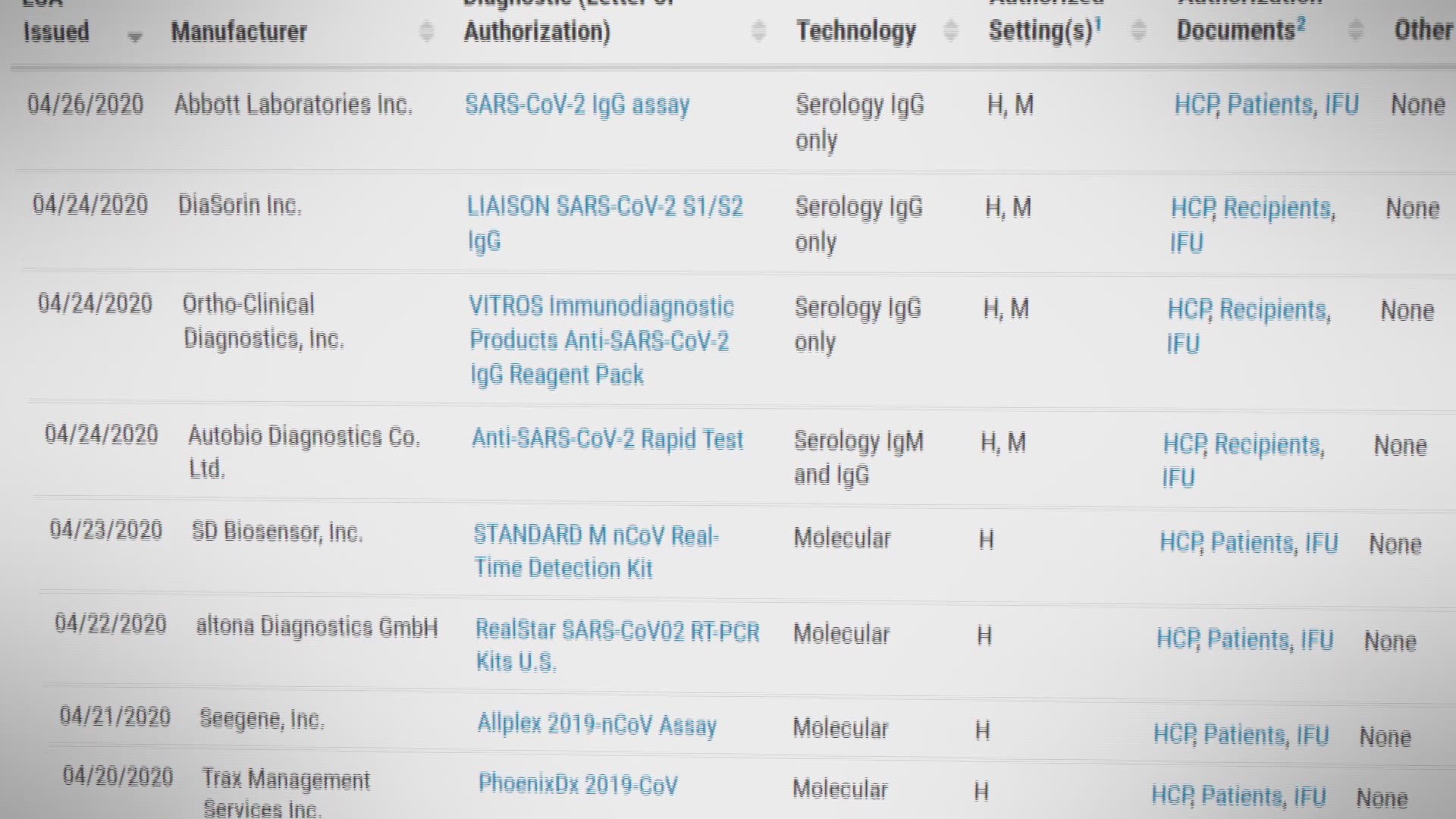
Is the abbott rapid covid test fda approved. 268 Zeilen The FDA expects that the tests on this list will not be distributed unless and until an EUA is issued for the test and the FDA may take additional actions as appropriate. Though the Abbott rapid test is one of over 100 COVID-19 diagnostic tests to receive FDA emergency use authorization during the pandemic President Donald Trump has featured the product in. FDA emergency use authorization for guided at-home use.
However CMS has indicated pdf icon external icon that CLIA will temporarily allow CLIA-certified laboratories and other testing sites to use SARS-CoV-2 point-of-care and rapid antigen tests on asymptomatic people for the duration of the COVID-19 public health emergency. All COVID-19 tests in the US. TPG recently reviewed Abbotts BinaxNOW COVID-19 Home Test which is now CDC-approved for travel to the US.
Each test comes with two tests per box and the company says to swab yourself twice within three days with at least 36 hours between. The ID NOW system processes a specimen in 15 minutes which enables. Abbotts rapid tests are among the most widely-used in the US with more than 200 million of our BinaxNOW and ID NOW rapid tests used in urgent care clinics doctors offices pharmacies nursing homes and schools since April 2020.
United Airlines confirmed that this particular test is compatible with the airlines Travel-Ready Center and American is accepting the test as well making it easy to link your results with your reservation. Food and Drug Administration FDA approved the rapid COVID-19 test for Emergency Use Authorization based on preliminary results of the study. The Food and Drug Administration FDA has approved the use of five rapid test kits for the detection of the coronavirus disease 2019 COVID-19.
Abbott Labs is a leading FDA approved laboratory with emergency use authorization from the FDA to administer the COVID-19 Rapid test. And United Airlines announced that this particular test is compatible with the airlines Travel-Ready Center making it easy to link your results with your reservation. Abbotts rapid COVID-19 test receives FDA approval for at-home use.
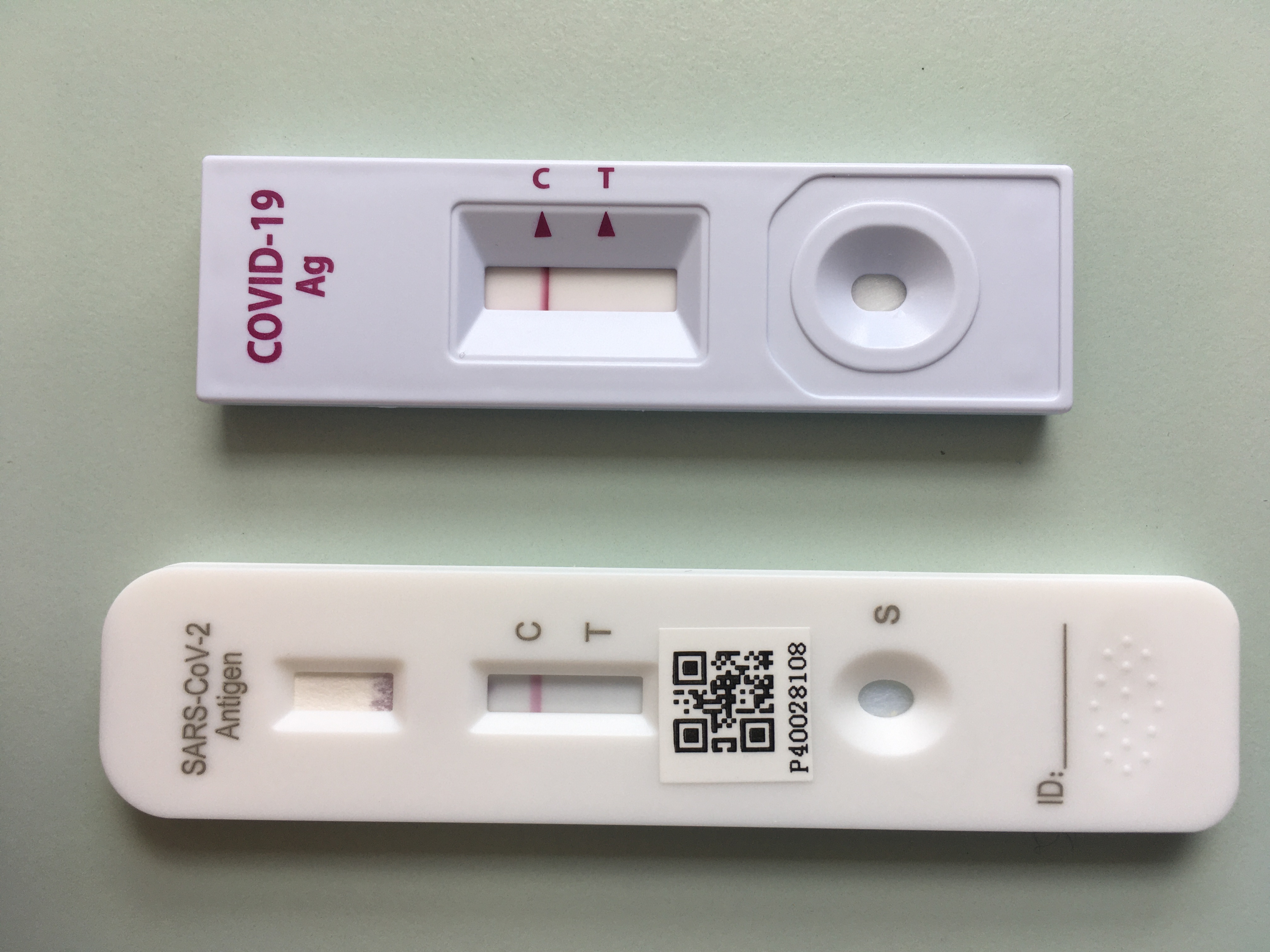
All of the FDA-authorized tests for current SARS-CoV-2 infection are for use on symptomatic people. On August 26 the US. This image provided by Abbott Laboratories in August 2020 shows the companys BinaxNOW rapid COVID-19 nasal swab test.
Given the simple nature of this test it is likely that. This test has been authorized for use in patients suspected of COVID-19 by their healthcare provider within seven days of symptom onset. These point-of-care test kits are registered for use in countries with reliable regulatory agencies such as China and Singapore the FDA said in a statement on Monday.
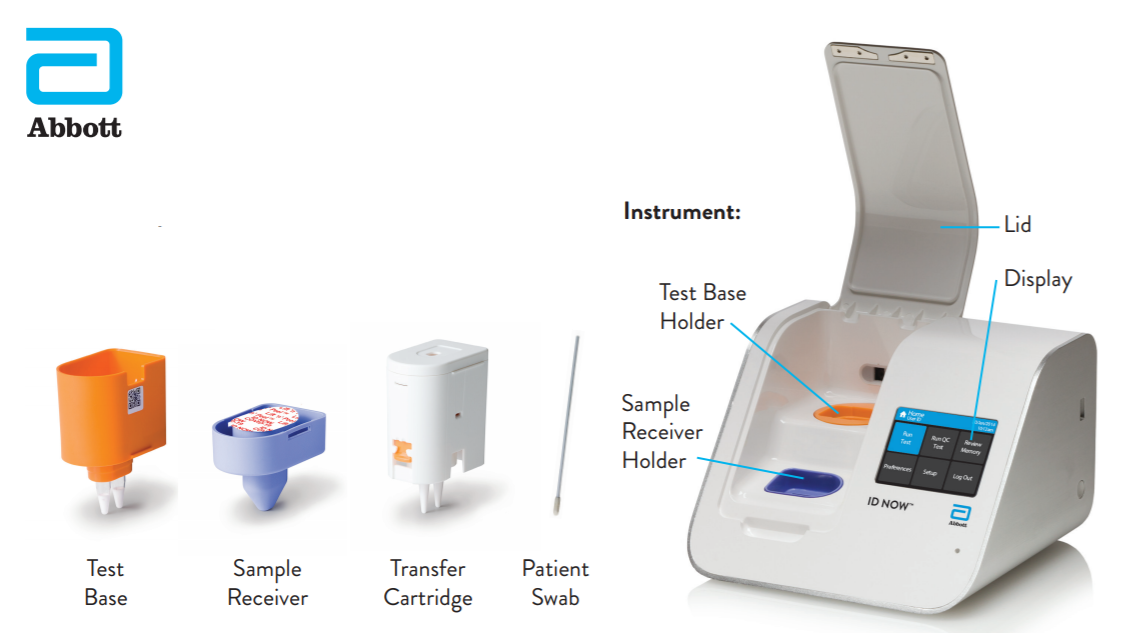
Dec 16 2020 at 343 PM. FDA-approved rapid US5 coronavirus test doesnt need specialty equipment. At the onset of the COVID-19 pandemic Duke Emergency Medicine partnered with Abbott Technologies as one of 19 sites within the United States to evaluate a rapid COVID-19 test that provided results within 15 minutes.
It was further updated to include reference that Abbotts BinaxNOW COVID-19 rapid test received US. 27 2020 HealthDay News -- The first rapid coronavirus test that doesnt need any special computer equipment to produce results was approved. The BinaxNOW COVID-19 Ag Card Home Test has not been FDA cleared or approved.
Food and Drug Administration issued the first emergency use authorization for a point-of-care COVID-19 diagnostic for the Cepheid Xpert Xpress SARS-CoV-2 test. These tests had been previously authorized by the agency some under different names to test those with COVID-19 symptoms but the actions this week authorize testing of asymptomatic individuals. A second Abbott rapid test also offers quick results.
The ID NOW is a lightweight and portable instrument the size of a toaster a molecular test which detects genetic material and allows testing to occur on site in order to get rapid results. Have been authorized under FDA Emergency Use Authorization. HealthDay Reporters THURSDAY Aug.
White House Receives New Fda Coronavirus Test Guidance 13newsnow Com

Fda Investigates Accuracy Of Abbott S Rapid Coronavirus Test Marketwatch

Abbott S Covid 19 Antigen Test Receives Fda Emergency Use Authorization

Fda Authorizes 2 Rapid At Home Coronavirus Tests Wlrn
Covid 19 Rapid Antigen Test Wikipedia
Medconsult Bangkok Medical Clinic Posts Facebook
Abbott S Binaxnow Covid 19 Rapid Test Receives Fda Eua

Fda Approves 5 Covid 19 Test That Takes 15 Minutes For Results Abc10 Com
Instant Results From Abbott 39 S Covid 19 Point Of Care Tes

Abbott S Binaxnow Rapid Antigen Self Test Receives Fda Eua Pharmalive

Abbott Defends Rapid Covid 19 Test With Interim Trial Results Massdevice

Covid 19 Antigen Tests Will Tremendously Increase Testing Ability In The Us

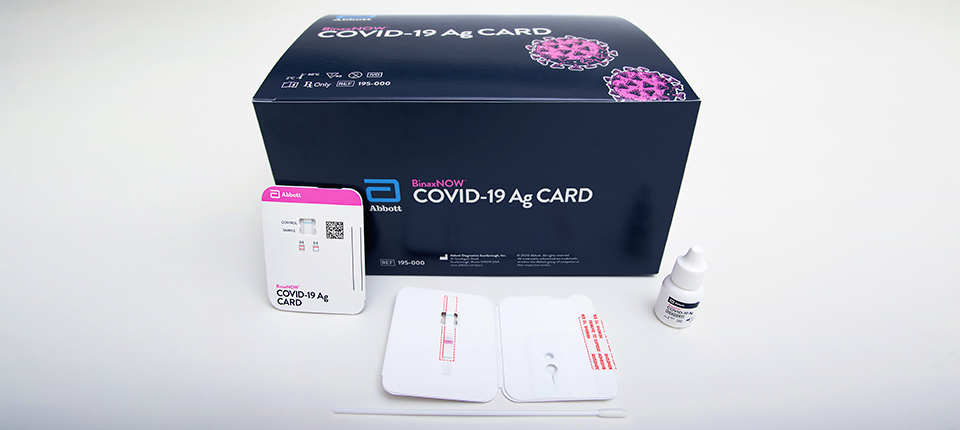
Binaxnow Covid 19 Antigen Self Test

Binaxnow Covid 19 Antigen Self Test

Study Raises Doubts Over Effectiveness Of Abbott Laboratories Rapid Coronavirus Test
Covid 19 Testing Is Needed On A Mass Scale Abbott Newsroom

Abbott Id Now Covid 19 Detection Test System Us
Abbott Binaxnow Covid 19 Antigen Self Test Kit 2 Ct King Soopers

Abbott Covid 19 Rapid Antigen Test Kit Icmr Approved At Rs 90 Piece Delhi Id 23448253330

Post a Comment for "Is The Abbott Rapid Covid Test Fda Approved"